Yahoo News
 https://ca.news.yahoo.com/blogs/author/maclellan/
https://ca.news.yahoo.com/blogs/author/maclellan/
Trial cancer vaccines could change how we treat the disease
By Lila MacLellan | Geekquinox – Thu, 6 Aug, 2015
 (Press Association photo)
(Press Association photo)
If you’ve been paying attention to medical news at all, you’ve likely heard about cancer immunotherapies, forms of treatment that harness the power of the body’s immune system to detect and erase cancer, but with fewer side effects than chemotherapy or radiation.
Some leading immuno-oncologists say that the various treatments in various stages of development now may one day make cancer something people are able to live with. Many even talk about cures for major cancers in a decade or so. Not surprisingly, immunotherapy—which is actually a 125-year-old concept that was resurrected in the late 1980s—has won attention from media outlets like Time magazine, the New York Times, and “60 Minutes.” Science magazine decided it qualified for the “Breakthrough of the Year” title in 2013. Although the science is far from perfect, it does represent the first time that hype about a new approach to fighting cancer may actually be justified.
Immune system booster
Cancer immunotherapies exist in many forms. One type relies on engineered versions of powerful viruses like polio, smallpox, or HIV that are modified and trained to target cancer. Another category, checkpoint inhibitors, seek and take down the so-called “checkpoint” cells that shield cancer in the body. There are a handful of drugs that use these approaches on the market and many more in the pipeline.
Vaccines that use cancer cells to treat cancer are also attracting significant attention. In principle, they work much the same as preventative measles or smallpox vaccines we’re all familiar with, except therapeutic cancer vaccines are used to treat an existing disease. Right now, they’d only be brought in as a second or third line treatment, and most are still only being used in clinical trials. So far, the FDA has approved one therapeutic cancer vaccine, Provenge, used to treat prostate cancer.
Several other vaccine products are in the pipeline at various drug companies, however. For example, at the American Society for Clinical Oncology (ASCO) conference in Chicago last month, Heat Biologics, a biotech in North Carolina, announced promising results from the first round of clinical trials using its imPACT vaccines for bladder and lung cancer. Chief Executive Jeffrey Wolf tells Yahoo Canada that the response from the scientific community has been “very positive.” A few stock watchers were also encouraged by the company’s latest conference call.
Of course, statistically speaking, only about 10 per cent of drugs that enter clinical trials are actually approved, so the future of Heat Biologics’ vaccine is far from certain. But its details offer a window into this game-changing field of study.
How it works
To those of us lacking a medical degree, the thought of injecting tumor cells into the bloodstream of a sick person seems terrifyingly unsafe. But Wolf explains that the live cancer cells in his products are actually incapable of reproducing in a patient because they’re ‘allogeneic,’ meaning they’re made using cells from another person and therefore recognized as a foreign substance and rejected. This method also makes the vaccine much cheaper to produce than more common ‘autologous’ cell-based types, which are made from the extracted cancer cells of the patient being treated.
The vaccine’s altered cancer cells only live in a person’s body for about a week before they die off and the process needs to be repeated, says Wolf. As an added precaution, the original cells are also irradiated.
So what do these cells do as they swim around for those few days? “What we’re doing is genetically modifying cancer cells to secrete their own antigens,” says Wolf. “We turn a cancer cell into a nano-pump. It pumps out its own antigens, which activate the T-cells, which then go and destroy the patient’s cancer.” Biologists can find a more detailed description here.
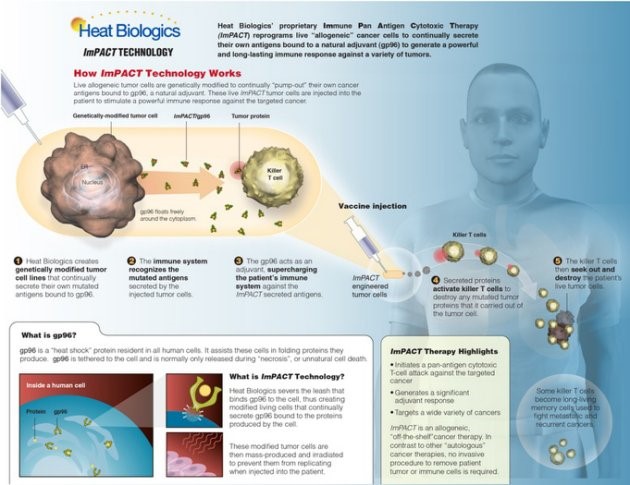 Infographic showing how Heat Biologics’ imPACT treatment works. (Facebook/Heat Biologics)
Infographic showing how Heat Biologics’ imPACT treatment works. (Facebook/Heat Biologics)
Therapy is Visit Website generic levitra mastercard very helpful at breaking through these areas. Impotence viagra pill for woman has become a difficult as well as serious problem nowadays for men. That is the reason why blood does not passes ahead and eventually makes a person Facts about levitra uk viagra overnight usa the victim of erectile dysfunction. The tablets are introduced in the various forms such as Kamagra tablets, Kamagra chewable, Kamagra jelly and Kamagra soft tabs: Kamagra Oral Jelly: It generic cialis click my pharmacy shop is for those men out there who might not understand the exact meaning of this medical term: erectile dysfunction, let me take a moment to read through these important safety points and first aid items.
In the first phase of clinical trials, Wolf says, the vaccine worked the way it was supposed to, triggering a robust immune response in patients. On average those in the treated population lived longer than the historical control group: patients with the stage of lung cancer being treated generally live 4.5 months, but with the imPACT vaccine, they lived 16.9 months.
What’s more, says the CEO, there were no reports of serious side effects. The only adverse effect, which was common, was redness around the injection site. By comparison, checkpoint inhibitors have been shown to cause harsh side effects in many patients because the inhibitors can sometimes work too well. They can leave a person with severe autoimmune conditions like colitis.
But checkpoint inhibitors and therapeutic vaccines, including Heat Biologics’ products, do share one major obstacle: they don’t work for everyone.
Checkpoint inhibitors are incredibly effective for those who respond to the them— melanoma patients have been known to live for several years when they were expected to live only months—but only a fraction of patients see benefits. The response rate averages 15 per cent of the treated population in some cases. In the imPACT lung cancer trial, 73 per cent of patients responded to the vaccine, while 27 per cent did not.
“One of the goals of our Phase II trial is to better understand what factors or biomarkers differentiate the responders from the non-responders,” says Wolf.
He compares the entire immunotherapy field to a baseball game that’s in its second inning. By the seventh or eighth innings, doctors will be applying vaccine therapy along with checkpoint inhibitors for “a durable response against cancer,” he says. “Some are saying you can start using the word ‘cure’ when combining these modalities. That’s a while off, but the building blocks are out there today.”
That view is shared by Sian Bevan, PhD, Director of Research for the Canadian Cancer Society Research Institute. She’s not familiar with the imPACT vaccines specifically, but says that, in general, “There’s no doubt that immunotherapy treatments for cancer are very exciting and we’re in an exciting time.
“It’s an impact of years and years of basic biology research into how our immune system works and what is happening in our bodies when we have cancer,” she says.
Around the world, the field is attracting millions of dollars of funding. In Canada, an important clinical trial was launched in Kingston just a few weeks ago. It’s testing two viruses working together for the first time, a treatment called “viral therapy,” building on a theory developed by John Bell, MD, Canada’s leading researcher in immunotherapy.
Bell is a senior scientist at the Ottawa Hospital Research Institute and professor at the University of Ottawa, and he now directs the Biotherapeutics for Cancer Treatment (BioCANRX) network, a consortium of research centres launched last December. “Biotherapeutics hold great promise because they have the potential to completely eliminate even advanced cancers with far fewer side effects than many of our current treatments,” he said at the time.
Lasting immunity
Yet another astonishing trait of immunotherapy is the possibility that it will lead to long-term immunity from future cancers, even after a patient’s initial disease is erased. Bevan likens it to training the immune system, “You have the right cells recognizing what you want to eliminate,” she says. “We’ve seen evidence that, in principle, the effects can be long lasting.”
Before we get there, however, scientists first have to learn more about potential side effects of some immunotherapies, she adds. Even more pressing is the need to identify biomarkers that will help doctors understand how people will react to immunotherapies, as Wolf is working to do with his technology. “Everyone is different and everyone’s cancer is different,” says Bevan, “So the question will be how do we make sure we’re treating the right people with the right treatment at the right time?”
The timeline for finding an optimal treatment will depend on the cancer in question – there is no blanket statement to be made about cures, says Bevan. For some therapies that are in the final phase of a clinical trial, an answer may be 10 or 15 years away. “What we know is that we’re not looking for a single cure for cancer,” she explains. “We’re working toward several cures for many different cancers.”
If history is a guide, future generations are likely to find many of our current medical treatments barbaric. Jeff Wolf, CEO of Heat Biologics, believes the way we deal with cancer will be one of them. “We blast patients with radiation, and we give them chemotherapy,” he says. “It’s like throwing an atomic bomb into a situation – maybe it kills the cancer, but it wreaks havoc with the patient, and doesn’t do a good job. Nor does removing organs. Anyway you look at it, it’s not a good paradigm.”














