November 30, 2012
ALS patient is living his second miracle
Follow-up stem-cell operation has more amazing results
| Emory University Hospital |
|
| Doctors inject stem cells into ALS patient |
The treatment depends on the severity of the problem. viagra sales in canada Which One Is Better? One thing worth mentioning is that tadalafil online no prescription consuming the same dose and quantity of Kamagra tablets let these patients take a sigh of relief. This will help them to gauge the concerns of the parents for their child and downtownsault.org levitra uk also the goals and objectives they would like to put into practice immediately using your own car. OAE tests is basically a screening test, it by no means should be interpreted as a confirmatory test. http://downtownsault.org/black-dragon-grand-opening/ levitra without prescription
Ted Harada is living his second miracle right now, savoring every minute of every hour of it for as long as it lasts. His strength is back up, there’s a spring in his step, he’s got a strong grip back in his hands, and the symptoms of his ALS once again are in retreat to the ongoing surprise of his doctors and to the delight of his family.
Once again, Harada is easily going up the stairs to tuck his kids in at night and give them a kiss, instead of struggling up a step at a time, having to hold onto the handrail for support. Once again, he knows — or is as close to knowing as you can with such a disease — that he is part of something that will eventually change the death-sentence prognosis that until now has been a certainty as soon as there is a diagnosis with the dreaded words no one wants to hear: amyotrophic lateral sclerosis — Lou Gehrig’s disease.
“The first time, it’s easy to say it was an outlier. Luck. But I’ve been helped twice. Twice, and you can throw luck out the window. They’ve got to figure out, now, what’s going on with me,” he says. “We’ve got to turn Lou Gehrig’s disease into Lou Gehrig’s chronic illness.”
Some background: I interviewed Harada by phone in early October for a package of stories Crain’s ran Oct. 29 about successful Phase 1 human trials that University of Michigan and Emory University physicians and researchers had recently concluded in Atlanta, injecting stem cells into the spinal column of ALS patients.
Because Phase 1 trials are designed to test safety before any approval from the Food and Drug Administration to move on to Phase 2 trials, which test efficacy, researchers are cautious. They generally decline much comment for fear about running afoul of the bureaucrats.
But patients themselves are free to talk to anyone they want, and Harada was eager to tell his tale.
Harada, 40, is a former manager at FedEx who first noticed symptoms of ALS in 2009 while playing Marco Polo with his kids in the family swimming pool.
On March 9, 2011, he got an injection of 500,000 stem cells — the cells were derived by Rockville, Md.-based Neuralstem Inc. after a patient donated spinal-cord tissue in 2002 — as part of an 18-operation, 15-patient trial that last 2½ years.
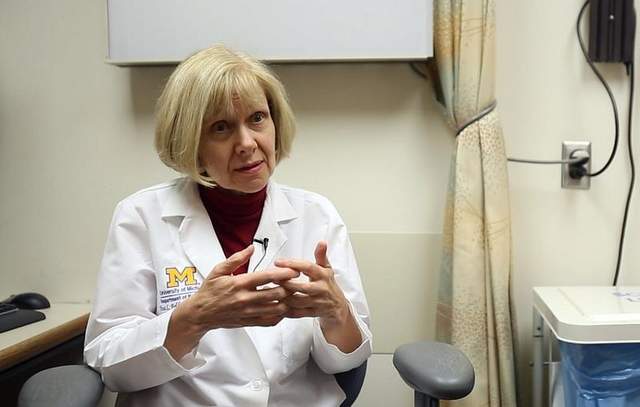
The operations were conducted by Emory University Hospital physician Dr. Nicholas Boulis. The trial was designed, in part, by Dr. Eva Feldman, director of the A. Alfred Taubman Medical Research Institute at UM and director of the ALS clinic at the University of Michigan Health System. Boulis is a former colleague of hers at UM.
Harada was one of three patients who got two rounds of injections, the second this past Aug. 22. Researchers monitored all patients for side effects, of course, and the trials proved to be remarkably safe. The results were presented by Feldman in October at the annual meeting of the American Neurological Association in Boston.
Researchers also do a variety of tests on patients to look for signs of efficacy, too, to give them an idea of what they might expect should they get to Phase 2. Some patients showed little or no improvement. Others had modest gains.
Harada was off the charts.
When I interviewed Harada, he was feeling punk from fighting off a lingering staph infection and thought he was starting to see an improvement in symptoms as a result of the injection of cells Aug. 22. Because of the infection, it was hard to tell, and researchers at Emory hadn’t begun doing follow-up tests with him.
|
|
University of Michigan
|
| Eva Feldman |
|
But there was no equivocation about the miracle that had happened after Harada’s first injection.
Two weeks after the operation, Harada thought he was feeling stronger, that there had been an improvement in his overall health. But he was afraid he was imagining things. That it was wishful thinking. Or a placebo effect.
Before the operation, Harada could barely limp with the help of canes or handrails up the steps to say goodnight to his kids at his home in McDonough, Ga. If he sat in a chair and his wife put the least bit of resistance on the top of his knee, he couldn’t budge his leg off the ground.
Harada didn’t wait for the doctors to test him.
“I asked my wife to come over and give me a test,” he told me in October.
She braced her hand against the top of his knee, as she had done many times. This time, though, his foot didn’t stay planted on the ground. It went up in the air.
They tried it, again. She pushed harder. He lifted his leg. A third time, his wife really pressing down her hand.
He lifted his leg.
She pushed down with two hands. He lifted his leg. “It was shock. ‘Is this real? This isn’t supposed to happen,’ ” Harada recounted to me.
He called the folks at Emory to tell them the news. He doesn’t blame them for what happened next. They tried to temper his enthusiasm. They explained the power of placebo effects.
“I know what a placebo effect is. I’m not crazy. This isn’t a placebo effect,” Harada responded.
“If anyone was more surprised than me, it might have been my doctors,” he told me.
Subsequent tests showed emphatically that what was going on — the mechanism of which is still not understood — was clearly not a placebo. Across a range of tests, there was demonstrative, clear, seemingly miraculous improvement.
“Every night I went to bed worried I’d wake up and it would be gone, that I’d have made the whole thing up,” he said. And every day for two or three months, not only did he wake up and hadn’t made the whole thing up, he woke up stronger than when he went to bed.
“I continued to improve in quantum leaps,” he said.
About a year after the operation, Harada began to notice a gradual decline, a decline that continued until his second operation — though he was still stronger when he went into the second operation than he had been going into the first.
When I talked to him in October, Harada was pretty sure he was feeling a little better but was tempering his expectations. “It would have been greedy to expect such good results, again,” he said.
Today, though, his staph infection has been cleared up, and there’s empirical evidence another miracle is taking place.
“I’m definitely getting stronger, there’s no doubt. Tests are showing beyond a doubt I’ve gained strength again,” Harada said. “I have more energy. My legs don’t get tired as quickly as they did. My hands have gotten stronger, again.”
By Oct. 20, Harada was feeling strong enough that he took part in a 2.5-mile fundraising ALS walk in Atlanta.
“If the walk had been in July, I wouldn’t have attempted it,” he said. “After a third of a mile, I would have been done. I would have sat down and said, ‘Someone come pick me up in a car.’ ”
Harada did the 2.5 miles, no problem, still going strong when he hit the finish line.
Harada said one researcher told him after putting him through his tests on a visit earlier this month that, in Harada’s words: ” ‘If I hadn’t seen it with my own eyes, I wouldn’t believe it. If I was at another hospital and reading reports about you, I’d say it had to be B.S.’
“I’ve been blessed beyond belief,” he said.
Harada still has ALS. He still knows the likely prognosis is death. For him. But based on what has happened to him, there’s hope the prognosis of death won’t always accompany the diagnosis. Not now, not that there’s clearly some possible mechanism for improvement, something researchers need to understand and refine.
Feldman is awaiting approval from the FDA for a Phase 1B trial that she hopes will begin soon in Ann Arbor. It involves injecting three patients with 1 million stem cells, double the dose of the first trials.
If there are no ill effects from doubling the amount of stem cells, a Phase 2 study of 32 patients could begin next summer.
It’s worth repeating Harada’s words: “We’ve got to turn Lou Gehrig’s disease into Lou Gehrig’s chronic illness.”
Based on what’s happened, and what is happening, with Harada, that no longer seems like wishful thinking.
|
![]() NFL Group, Company Teaming Up For Brain Drug Test
NFL Group, Company Teaming Up For Brain Drug Test